Research
PROVEN TO RESET PANIC, SPIRALING STRESS, AND RESTORE CALM — SAFELY
Auricular vagus nerve stimulation (aVNS)—the foundation behind VeRelief Prime—has been rigorously studied across thousands of sessions.
Clinical trials show it’s safe, fast, and effective at calming the nervous system, even during acute stress and panic episodes.
SAFETY PROVEN IN THOUSANDS OF USERS
Across 150+ participants in placebo-controlled studies, there was no increased risk of side effects between real VeRelief stimulation and placebo.
In over 18,000 sessions of vagus nerve stimulation across healthy, neurological, and psychiatric patients, no serious side effects were reported (Berthoud & Neuhuber, 2000).
(source).In over 6,000 participants, there was no higher risk of adverse events for those receiving real versus sham stimulation.
(source).Among 1,300+ participants, minor side effects like tingling (18%) and mild headache (3.6%) were the only issues reported (Kraus et al., 2007)—no dizziness, sedation, or drug-like complications.
(source).
VeRelief offers fast recovery without compromising safety—even under extreme emotional states.
STUDY ONE
Engineering Faster Mental Recovery with Vagus Nerve Activation
Dr. Nicholas Hool, Dr. William J. Tyler, Dr. Sarah Wyckoff, and Dr. Taylor Hearn conducted a study aimed at developing a Targeted Neuroplasticity Training (TNT) method to enhance foreign language learning. They focused on optimizing noninvasive vagus nerve stimulation (VNS) techniques that are comfortable and suitable for everyday use in various training environments.
The researchers explored transdermal auricular vagal nerve stimulation (taVNS), which targets nerve fibers in the external ear, as a more comfortable alternative to other methods. They designed biocompatible, hydrogel earbud electrodes for unilateral or bilateral use, and evaluated their safety and efficacy across different frequencies and intensities.
The team also examined the impact of taVNS on autonomic physiology, attention, sensory gating, and plasticity using various measurements, including heart rate, heart rate variability, skin conductance, skin temperature, respiration rate, EEG, and pupillometry.
Read the safety and efficacy report for the first prototype study here
STUDY Two
Rapid Anxiety Reduction Under Pressure
POPULATION
18 competitive golfers during a 150-yard hitting task.
DEVICE AND TREATMENT DURATION
10 minutes of vagus and great auricular nerve stimulation on both sides of neck (bilateral stimulation).
RESULTS
36% Clinically Significant Reduction (36%) in state-anxiety in active treatment group after single 10-min treatment. Only 18% reduction in placebo group.
Compare to 10 minutes with the top alternatives:
No differences in safety and tolerability between active and placebo treatments (Pain, dizziness, blurred vision, headache, skin itching or irritation).
Significant increase in quality of feel of each shot in active group only.
STUDY Three
Breaking the Panic Spiral in PTSD and Panic Disorder Patients
POPULATION
24 patients with self-diagnosed Post Traumatic Stress Disorder and Panic Disorder.
DEVICE AND TREATMENT DURATION VeRelief handheld device
5-minutes of VeRelief treatment on each side of the neck for a 10-minute total treatment time.
Note: While these studies utilized 10-minute protocols for comprehensive data collection, VeRelief Prime's dedicated Panic mode (Mode 5) is optimized based on these findings to initiate a nervous system reset and provide noticeable relief in as little as 3 minutes, specifically targeting acute episodes.
RESULTS
24% Clinically Significant Reduction in State-Anxiety in active treatment group after single treatment
31% Increase in Heart Rate Variability (rMSSD) in active group alone. 0% change in sham group.
100% Of Active Users reported treatment to be "Relaxing". 33% of sham participants found treatment to be relaxing.
No differences in safety and tolerability between active and sham treatments (Pain, dizziness, blurred vision, headache, skin itching or irritation).relaxed than sham participants.
CASE STUDY
Stopping Panic Before It Spirals
POPULATION
Individual 23-year-old male patient living with diagnosed Panic Disorder and Post-Traumatic Stress Disorder
protocol
Use 2x per day for 8 weeks. After 8 weeks of using the devices, patient was asked to rate each statement as Strongly Agree, Agree, Neutral, Disagree, or Strongly Disagree.
results
"I feel better after using the device"
Strongly Agree 63% of the time
Agree 37% of the time
"I feel better having access to this device in case I have symptoms"
Strongly Agree 25% of the time
Agree 75% of the time
"The device helps me relax"
Strongly Agree 37% of the time
Agree 63% of the time
"The device decreases the severity of my symptoms"
Agree 75% of the time
"The device is easy to use"
Strongly Agree 12.5% of the time
Agree 75% of the time
Patient Statement: "It has stopped some panic attacks from getting worse and [felt like] I was melting into my bed. Complete mental and physical relaxation."
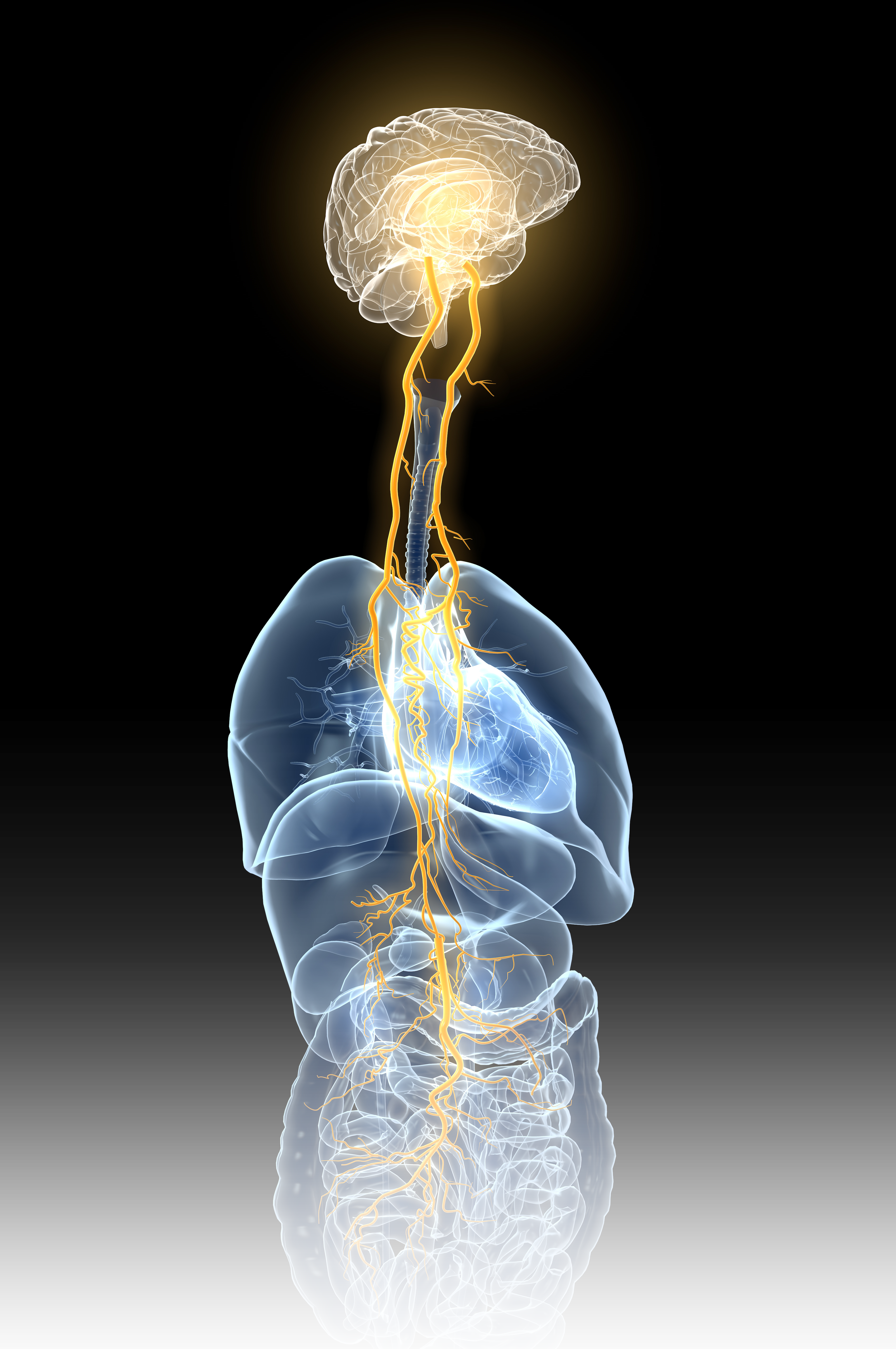
The Vagus Nerve
Your Built-In Panic Reset
The vagus nerve — your body’s ultimate power line — connects your brain to nearly every major organ, earning its name from the Latin word for “wandering” as it weaves through your entire system.
Your bodies vagus nerve is the vital switch that calms your system, releasing serotonin and acetylcholine to soothe panic and restore natural balance — the way your body was designed to heal.
The Vagus Nerve
Subscribe to stay updated.
On our product development efforts, learn about case study opportunities, chances to win free products, and other news and insights.

